The AcQualis™ CAPA Application
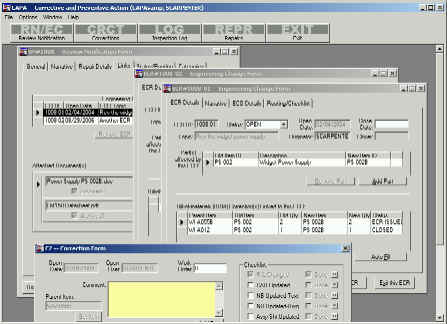
CAPA parent screen showing an RN, 2 ECR’s
and 2 Corrections
CAPA-centered quality software for medical devices
The AcQualis™ application handles CAPA (Corrective And Preventive Action) events
in manufacturing, for compliance with ISO 9001/2000, FDA and CE medical device
requirements, including 21 CFR Part 11 requirements for electronic documents.
Key Benefits
 | Manages CAPA (Corrective and Preventive Action) forms |
 | Manages engineering changes |
 | Manages document and bill-of-materials corrections |
 | Manages inspection logs |
 | Interacts with ERP inventory and bill-of-materials |
The AcQualis™ system is focused on...
 | Addressing the special significance of CAPA for
FDA-regulated device makers, leading quality-management teams to spend more focused
time on CAPA, generating clearer, fuller and more complete
documentation. |
 | Enhancing team collaboration - This system is the
only one we know that captures people's interactions so that in the
process of resolving an issue as a team they create a fully compliant
CAPA document, saving time and money. |
Presentation
Click here for a presentation that focuses on the unique advantages of
Commonwealth Software’s AcQualis™CAPA application:
Product Description
AcQualis™ is a collaborative application that gathers
critical quality-related
information from throughout the organization, contributed by people in a variety of roles. It interacts with the company’s
ERP system (Enterprise Resource Planning).
More specifically, the program manages a database made of several
different types of documents connected with Corrective and Preventive
Actions: (1) Review Notification (RN) forms - forms that trigger a
committee review, (2) Repair forms - handled as a subtype of RN, (3)
Engineering Change requests / Engineering Change orders, (4) Corrections
to Bills-of-materials and other documentation, and (5) Inspection Log
entries. The central CAPA document (RN) is more than just a
non-conformance form - it is not just negative - our application also
functions as a multi-ported, instantly accessible suggestion box, which
everyone in the organization is empowered to use, whenever there’s an
opportunity to identify a potential improvement in a product or
process.
Interlocking forms - Review Notification forms, Engineering
Changes and Correction forms have an interlocking relationship: A Review
Notification cannot be closed until its dependent Engineering Change(s)
are closed, along with other required actions. An Engineering Change can’t
be closed until its dependent Correction(s) are closed.
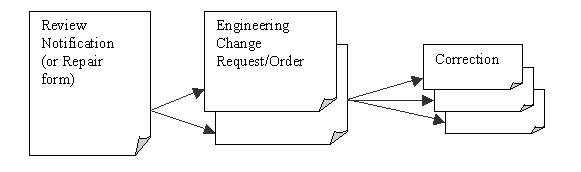
Correction forms are also used alone to
implement a simple but powerful workflow solution for correcting drawings,
bills-of-materials and other critical documents.

Focus on collaboration
- The application's distinctive features all contribute to enhance
collaboration; together these features present an exceptionally rich
document model:
 |
Threaded narratives - The threaded narrative
fields work like a “blog” or threaded discussion, and identify the
contributions of each participant with name and date, showing vividly how
the issue was tackled and resolved. Users compose comments in a separate
window where spell-checking is available. |
 |
Rule-based document life cycle management - A
document passes from one phase to the next in its life cycle when the
STATUS is changed, Configurable document life cycle means that status
identifiers are defined using a Status List Management screen. Each
change of status is validated using a set of Status change validation
rules. Rules are developed using a Rules Management screen. |
 |
Configurable checklists - Checklists are managed
both at the document level by clicking the Required box and
system-wide using the Checklist Management screen. Name and
date are recorded for each checkmark made. |
 |
Document-style concurrency –
several users can view a document while only one may edit it –
read/only users are notified when the document becomes available for
editing. |
 |
Attached Documents - Documents from any
Windows application (Word processing, spreadsheet, image, CAD) can be
attached in two stages: an active stage where they are editable
in place from any workstations that has the host application, and an archived
stage where they can be read but not changed. |
 |
Indelibility - Central to the design of this application is the
need to make it behave like indelible “ink on paper,” to disable
the computer’s normally-desirable ability to allow a user to change a
document without leaving a trace of the document’s state prior to the
change. CAPA uses many innovative methods to apply this constraint and to
communicate the state of the data to its users. |
Architecture - AcQualis™
is
developed using the Windows Multiple-Document Interface (MDI) and
ADO database connectivity. The CAPA data is kept in the form of a secure
relational database (MS SQL Server 2005). The ERP data comes via ODBC
or OLEDB from the ERP system’s
native database platform. Everything that persists in the application is
stored in the relational database, which handles the mechanics of sharing
data among multiple users and allows unlimited reporting and analysis
using the CAPA application’s own reports or such external tools as Crystal
Reports, Quantrix, Cognos, Microsoft Access, Excel.
|

|
|
